- 08 6180 7939
- andy@caaa.com.au
- Mon - Fri: 9:00 - 15:00 (WST Perth)

CAAA News
Navigating Shipping of Biological Substances
Facebook
LinkedIn
Navigating shipping regulations: Understanding infectious substances and biological specimens
In today’s rapidly evolving world, the transportation of infectious substances, biological substances, and patient specimens presents unique challenges and considerations for both the shipper and the air operator. As regulations continue to evolve, it’s essential for all organisations involved in shipping these materials to stay informed and compliant.
The transportation of infectious substances, biological materials, and patient specimens is governed by a web of regulations, including those of international bodies such as the World Health organisation (WHO) and the International Air Transport Association (IATA) and national authorities like the Civil Aviation Safety Authority (CASA). These regulations are designed to ensure the safe handling, packaging, labeling, and transportation of these materials to protect public health and safety. IATA issues their annually updated Dangerous Goods Regulations (DGRs) and CASA’s dangerous goods requirements are contained in CASR 92 and its associated materials.
See:
Dangerous goods and air freight | Civil Aviation Safety Authority (casa.gov.au)
Products – Civil Aviation Academy (caaa.com.au)
One of the key considerations when shipping infectious substances and biological materials is proper identification of the sample to be sent. The shipper must identify the classification of the sample for all subsequent elements of the process to be correct.
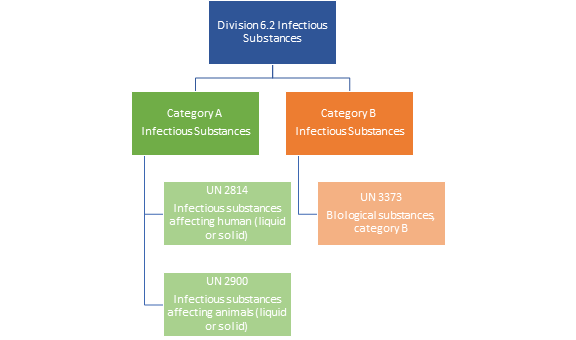
Infectious Substances (Division 6.2) are materials containing or reasonably expected to contain pathogens, such as bacteria, viruses, parasites, fungi, and prions, capable of causing disease in humans or animals.
Categories of Infectious substances:
Category A: Substances capable of causing permanent disability, life-threatening, or fatal disease in healthy humans or animals. Assigned to UN 2814 (human and animal diseases) or UN 2900 (animal diseases only).

UN 2814 and UN 2900 assignment is based on medical history, symptoms, local conditions, or professional judgment.
Category B: Substances not meeting Category A criteria. Assigned to UN 3373, known as Biological Substance, Category B.

There is a tendency to over classify infectious items. As it is much more difficult to ship Category A items this can create an issue for the shipping process if not necessary. The packaging is also far more restrictive and more expensive. Some airlines will not transport Category A items regardless of the item meeting the requirements of the IATA regulations. Some road couriers will also not carry these items to and from the airport. Due to amendments to classification over the past 5 years there is now a very limited number of substances that should be classified as Category A – most are now category B or in fact exempt. It is therefore worth the time and effort to correctly classify rather than over classify just to ‘be safe’.
Exemptions and non-regulated items include:
- Dried blood spots: not subject to IATA DGRs.
- Blood for transfusion and organs for donation: not subject to IATA DGRs.
- Low probability substances: not subject to IATA DGRs (e.g., foodstuffs, water, soil, dust samples).
- Live infected animals: strict conditions and approval by each applicable national authority (e.g., for Australia CASA) is required for air transport.
- Patient specimens: exempt if packed securely, marked as “exempt human specimen” or “exempt animal specimen”. Refer to IATA DGRs for packing instructions.
Correct classification is important. That is why all shippers of dangerous goods which include infectious substances, biological substances and the dry ice they may be contained in, must be trained every 2 years in a CASA approved course. Safe Transport of Infectious Substances – Civil Aviation Academy (caaa.com.au)
Another key element in the process is the correct packaging and labeling by the shipper. It’s crucial to use packaging materials that meet the requirements specified by regulatory authorities, such as UN-certified packaging for infectious substances in category A. Additionally, packages must be properly labeled with the appropriate hazard labels and shipping documents to indicate the contents and ensure safe handling during transit.
Furthermore, temperature control is often a critical factor when shipping biological specimens, particularly those that require refrigeration or freezing. Specialised packaging and transportation methods may be necessary to maintain the integrity of these specimens and prevent degradation during transit. Commonly water ice or alternatively dry ice is used to maintain this temperature control. If dry ice is used, regardless of the quantity, the shipper must be trained every 2 years in a CASA approved course, as detailed above.
In recent years, advancements in technology and logistics have led to improvements in the transportation of infectious substances and biological materials. For example, the development of temperature-controlled packaging solutions and real-time tracking systems has enhanced the ability to monitor shipments and ensure proper handling throughout the transportation process.
However, despite these advancements, shipping infectious substances and biological materials remains a highly regulated and complex process. Compliance with regulatory requirements is essential to avoid delays, fines, and potential risks to public health.
The shipping of infectious substances, biological materials, and patient specimens requires careful attention to regulatory requirements, proper packaging and labeling, and adherence to best practices. By staying informed and proactive, we can navigate these challenges and contribute to the safe and effective transportation of these essential materials.
Stay safe and compliant.
